ABSTRACT
In vitro drug screening using reliable and predictable liver models remains a challenge. The identification of an ideal biological substrate is essential to maintain hepatocyte functions during in vitro culture. Here, we developed a fiber-embedded polydimethylsiloxane (PDMS) chip to culture hepatocytes.
Hepatocyte spheroids formed in this device were subjected to different flow rates, of which a flow rate of 50 μL/min provided the optimal microenvironment for spheroid formation, maintained significantly higher rates of albumin and urea synthesis, yielded higher CYP3A1 (cytochrome P450 3A1) and CYP2C11 (cytochrome P450 2C11) enzyme activities for metabolism, and demonstrated higher expression levels of liver-specific genes.
In vitro metabolism tests on tolbutamide and testosterone by hepatocytes indicated predicted clearance rates of 1.98 ± 0.43 and 40.80 ± 10.13 mL/min/kg, respectively, which showed a good in vitro–in vivo correspondence. These results indicate that this system provides a strategy for the construction of functional engineered liver tissue that can be used to study drug metabolism.
MATERIALS AND METHODS
Poly(ethylene glycol)-poly(DL-lactide) (PELA; Mw = 42.3 kDa, Mw/Mn = 1.23) was synthesized in our laboratory as follows. DL-Lactide and poly(ethylene glycol) (PEG) were prepared by bulk ring-opening polymerization using stannous octoate as the initiator. To modulate the graft efficiency of lactose into fibers, 4-armed lactosylated poly( DL-lactide) (lac-PLA; Mw = 7.6 k Da, Mw/Mn = 1.32) was obtained by the polymerization of pentaerythritol and DL-lactide as described previously.
RESULTS AND DISCUSSION
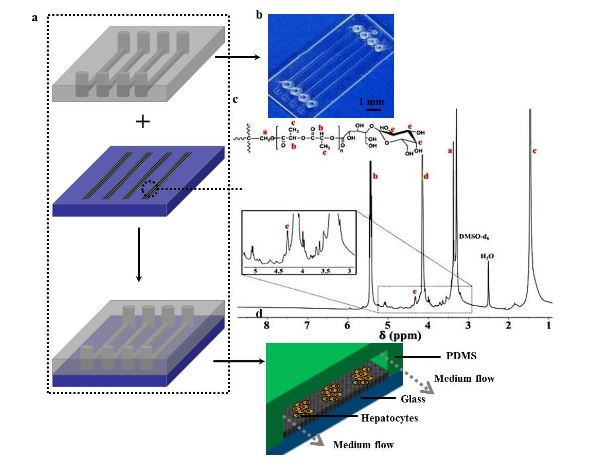
Figure 1. (a) Schematic illustration of a microfluidic device assembly
Figure 1 shows the schematic diagram of the process of patterning fibers to integrate with a microfluidic chip. The microfluidic chip consists of an upper PDMS channel, middle lac-PLA/PELA fibrous mats, and bottom glass (Figure 1a). Figure 1b shows an image of the PDMS channel, which includes 4 arrayed channels (10 mm long, 200 μ m broad); the space between parallel channels is 3 mm.
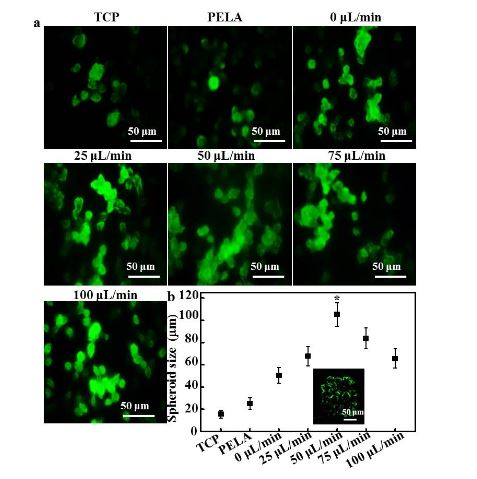
Figure 3. (a) Confocal laser scanning microscopy (CLSM) images of immunofluorescent staining of albumin by hepatocytes
Figure 3 shows the fluorescence images and size distributions of the spheroids formed in the microfluidic device. As shown in Figure 3a, rat hepatocytes cultured on TCPs and PELA showed a flat spread pattern and scattered morphology, probably due to the rapid loss of functions of isolated hepatocytes. The efficiency of spheroid formation by hepatocytes cultured under low flow (0 or 25 L/min) was not high during the culture period.
CONCLUSIONS
We report a fiber-embedded PDMS chip for uniformly sized hepatocyte spheroid formation and culture. Compared with other hepatocyte cultures, hepatocytes at a flow of 50 μL/min demonstrated significantly higher levels of enzyme activities and gene expression, better predicted drug clearance, and better in vitro–in vivo correspondence. The microfluidic device provides a useful platform toconstruct liver tissue in vitro, and it can serve as an in vitro testing model for the evaluation of drug metabolism.
Source: Sichuan Agricultural University
Authors: Yaowen Liu | Ke Hu | Yihao Wang